Neuroservices-Alliance recently partnered with “Biotech A company” to investigate a promising new proprietary compound. The objective of this preclinical study was to determine the compound’s effectiveness on spontaneous excitatory transmission in the lateral habenula, a key brain region implicated in stress and depression. To achieve this, our team utilized a restraint stress model in mice and conducted detailed electrophysiological recordings to measure the compound’s impact. This collaboration aimed to provide clear, data-driven insights into the compound’s therapeutic potential.
MATERIALS & METHODS:
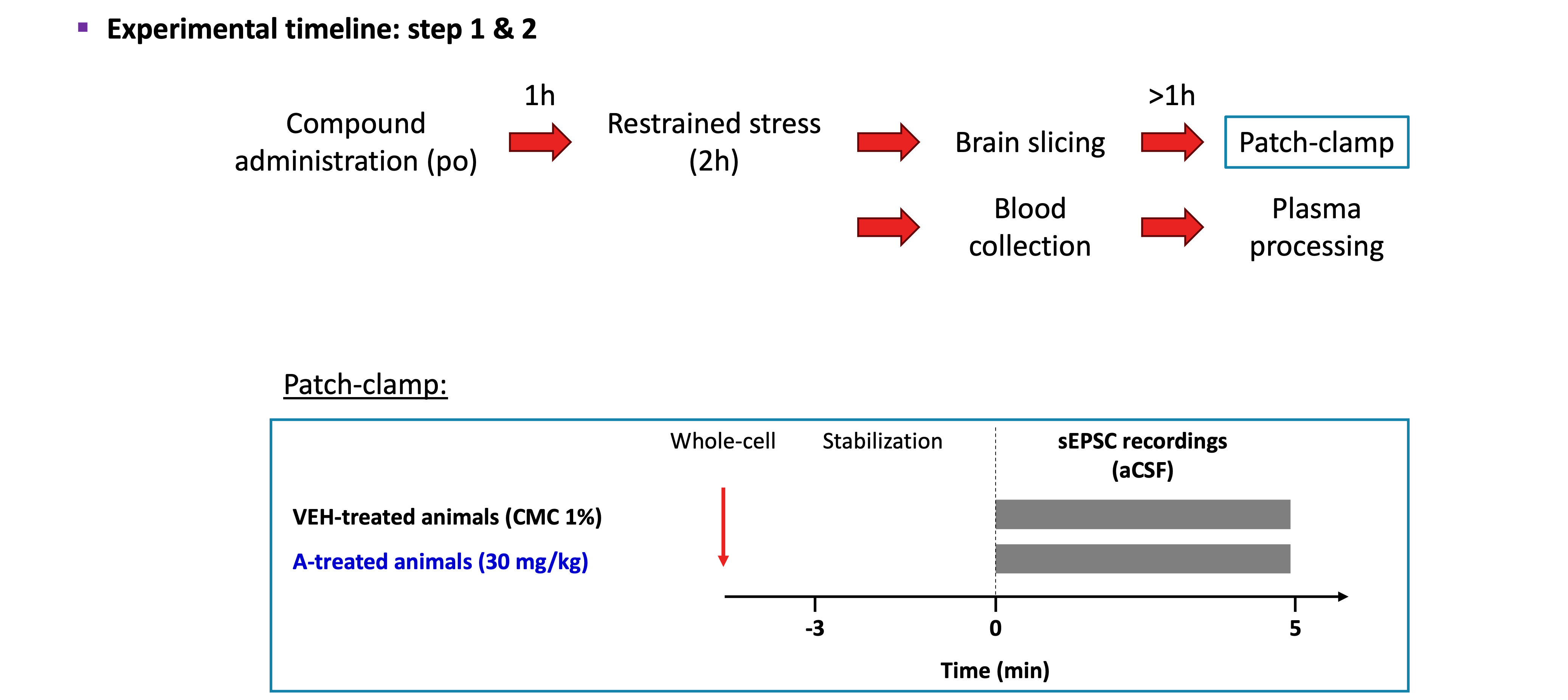
- Animals: 7- to 9-week-old male C57BL6/J mice were used for this study.
- Animals Dosing: Mice were administered with either vehicle (CMC 1%) or the test compound XXXXXX (30 mg/kg) via oral gavage (per os). Reference compound: Ketamine 10 mg/kg.
- Restraint stress: 60 min after the compound administration, the protocol of acute restraint stress was applied for 2 hours, following Kim and Han (2006).
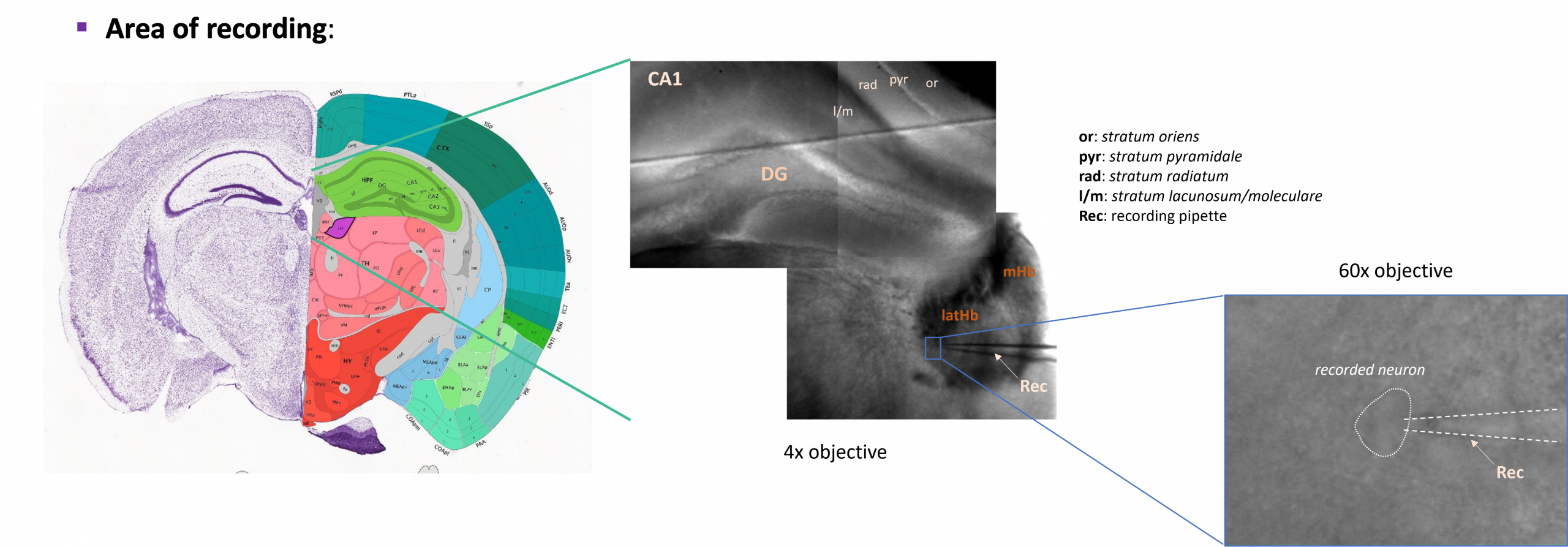
Lateral Habenula recordings:
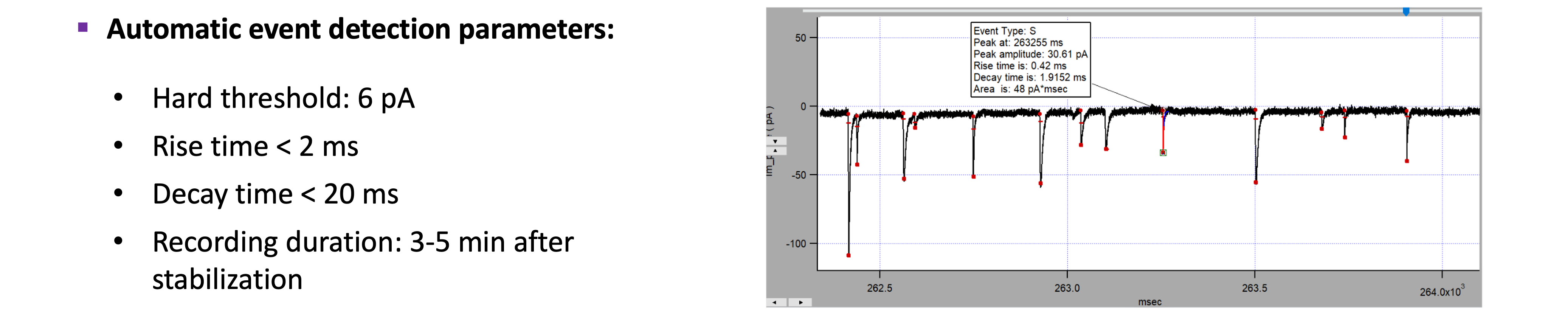
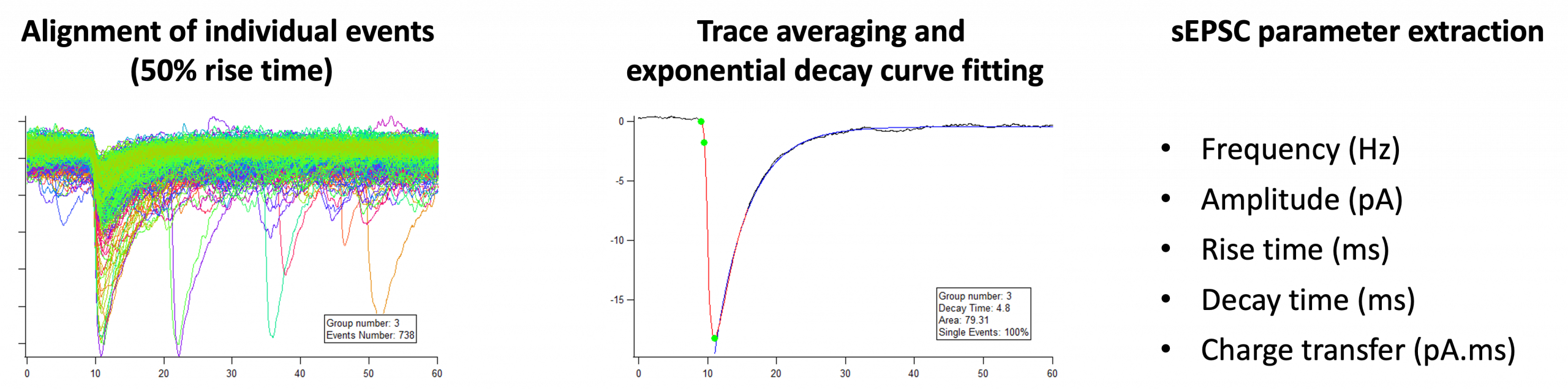
sEPSCs analysis :
EXPERIMENTAL PROTOCOL:
RESULTS:
Stressed vs Unstressed Animals
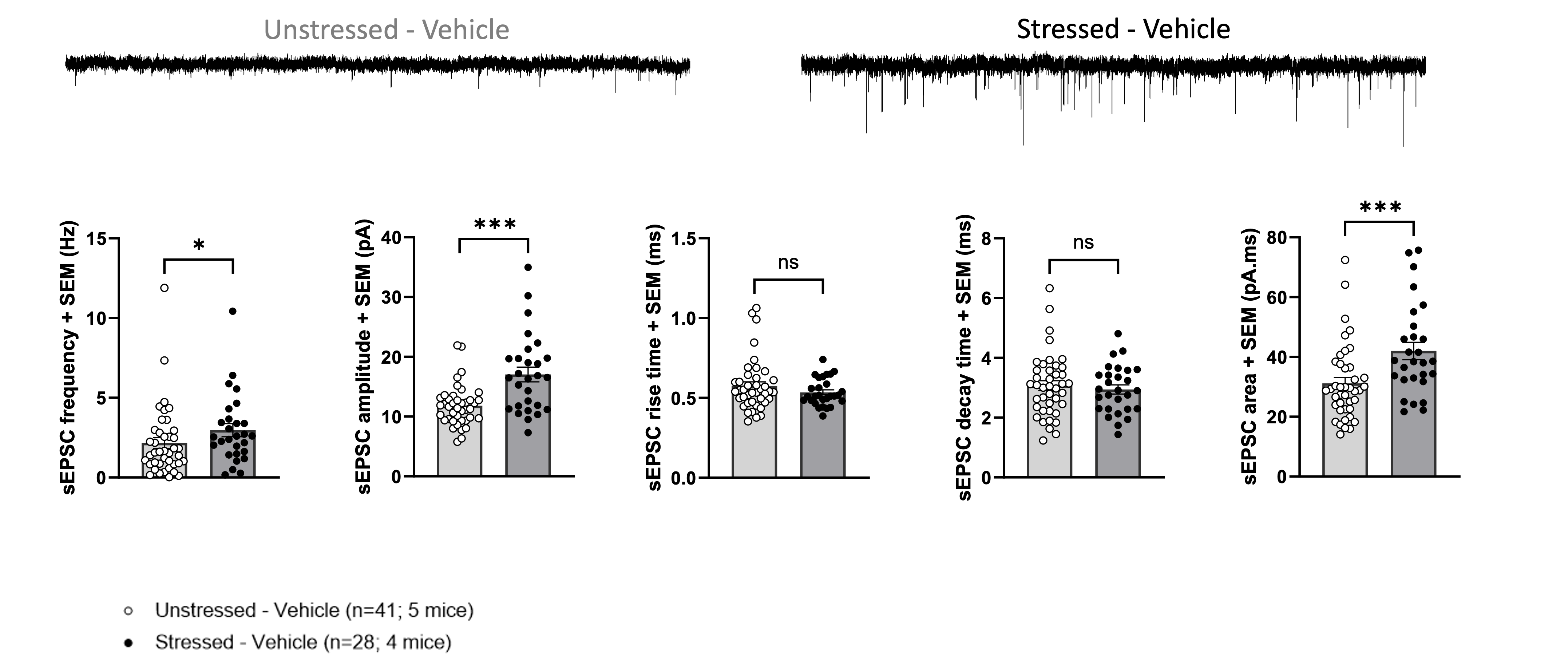
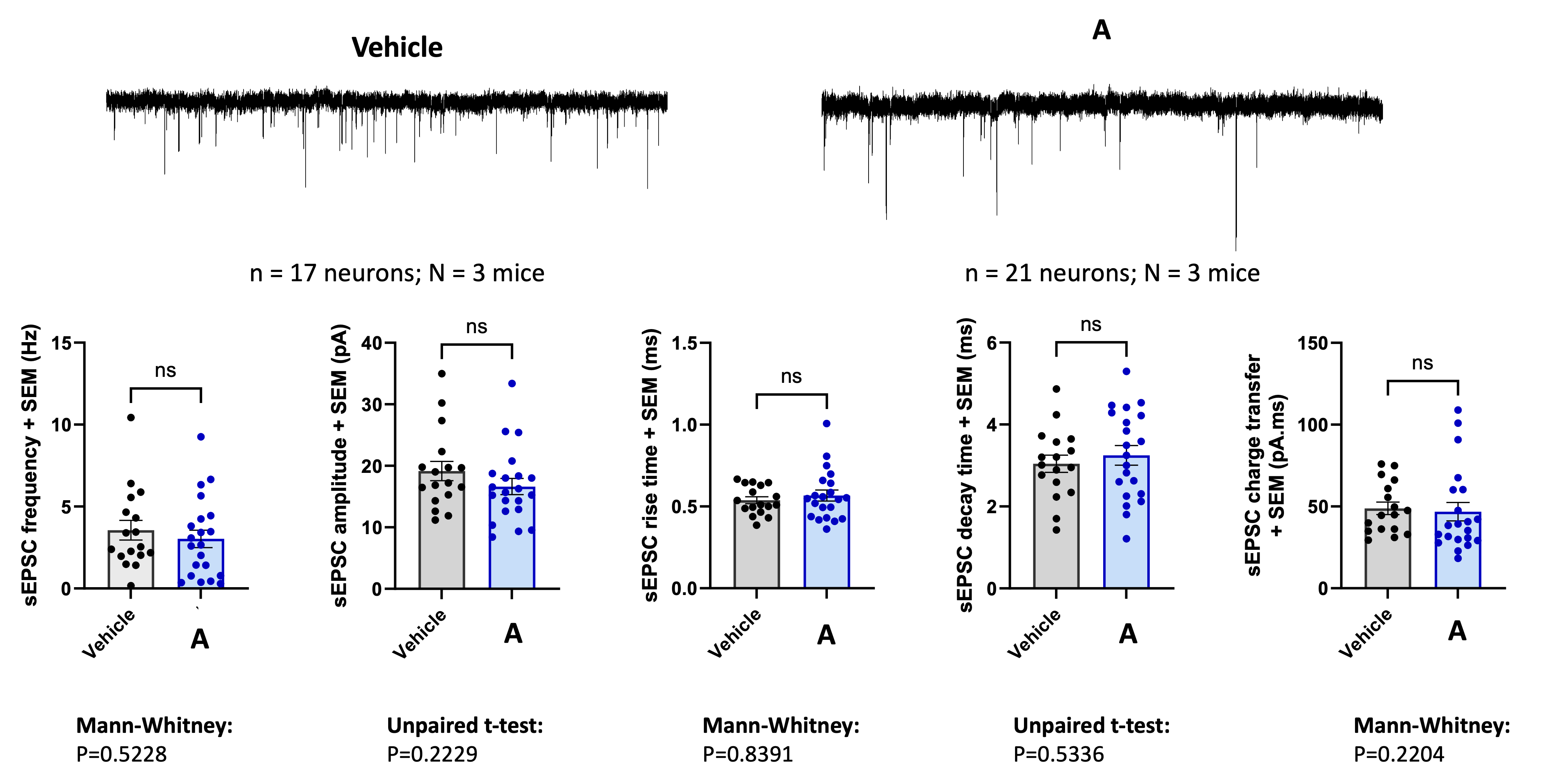
CONCLUSION:
While the results indicated that the test compound did not significantly alter neuronal activity in the lateral habenula in this specific preclinical model, this outcome is far from a setback. A “no-go” decision is as critical as a “go” decision, saving valuable time and resources. Our rigorous preclinical services provide pharmaceutical and biotech companies with the unequivocal evidence they need to make confident, informed decisions about their therapeutic candidates. This study serves as a testament to our commitment to delivering decisive results, ensuring our partners can strategically navigate the path to clinical trials and invest only in compounds with the highest potential for success.
Ready to bring this level of clarity to your own drug discovery program?
Contact our experts today to discuss your next project.
Send us an email
Go back